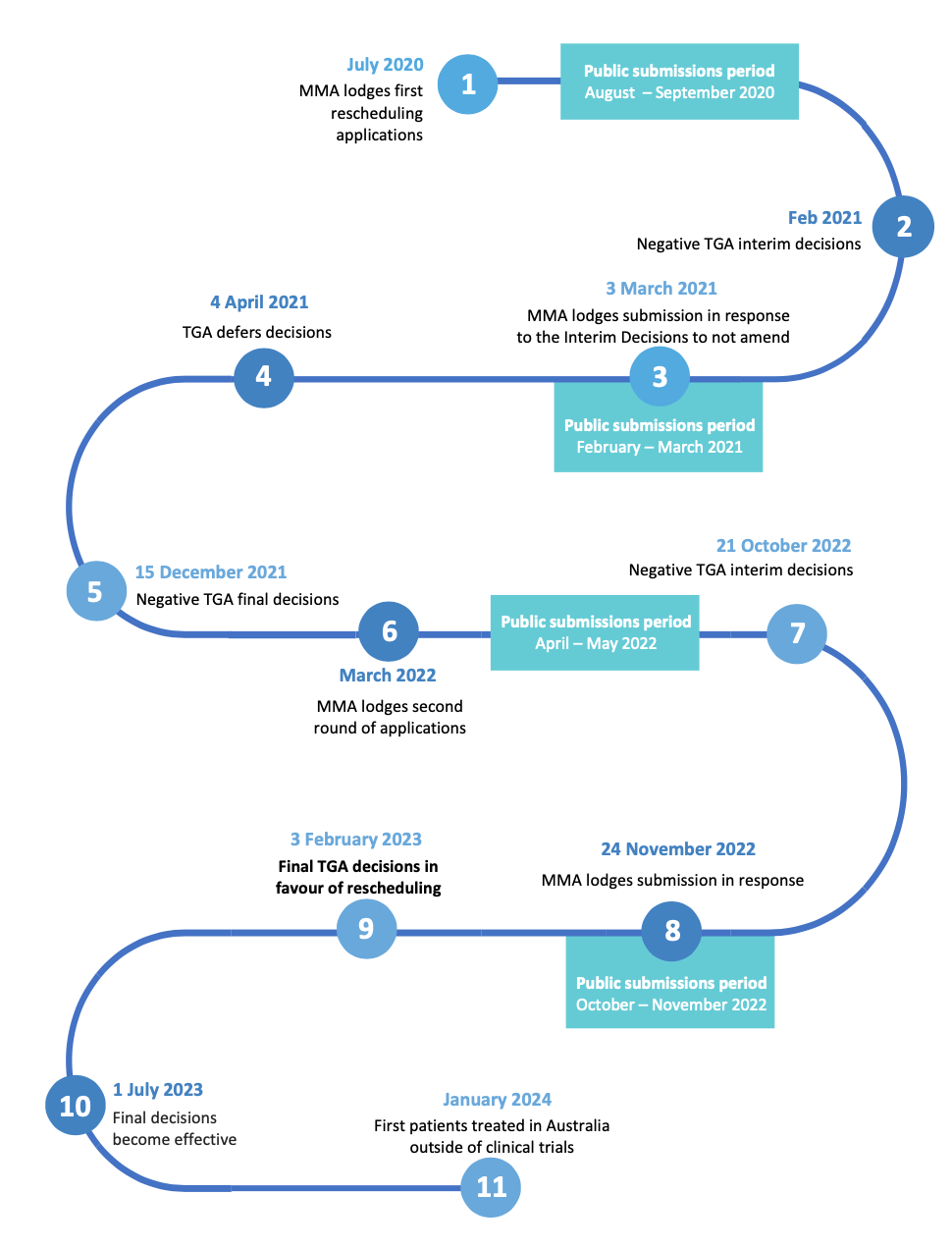
In March 2022 Mind Medicine Australia lodged new applications with the TGA for the restrictive rescheduling of the medical use of MDMA and psilocybin as part of therapy in medically controlled environments from Schedule 9 (prohibited substances) to Schedule 8 (controlled medicines) of the Poisons Standard.
In February 2023 the TGA approved Mind Medicine Australia’s application to reschedule the substances psilocybin and MDMA from Schedule 9 (prohibited substances) to Schedule 8 (controlled medicines) of the Poisons Standard. With this rescheduling, psychiatrists who have the requisite approvals are now able to utilise psilocybin for treatment-resistant depression and MDMA for PTSD. These changes came into effect July 1st, 2023.
This groundbreaking decision makes Australia the first country in the world to reschedule these medicines, and everyone at MMA would like to extend our deepest gratitude to everyone who took part in this process and helped to make it happen.
Our Successful Rescheduling Applications for the Medical Use of Psilocybin and MDMA:
Successful Rescheduling Applications for the Medical Use of Psilocybin
These were lodged with the TGA in March 2022 and can be found here.
Successful Rescheduling Applications for the Medical Use of MDMA
These were lodged with the TGA in March 2022 and can be found here.
The TGA's Final Decision to Reschedule MDMA and Psilocybin
The TGA's Final Decision to Reschedule MDMA and Psilocybin
The TGA Announced its Final Decision to Reschedule on 3 February 2023.
Our Media Release in response to the TGA Final Decision
Breaking News: The TGA Announces the Delegate’s Final Decision to Reschedule the Use of Psilocybin and MDMA for Medical Purposes
Read our media release here - 3 February 2023
The TGA’s Interim Decisions against Rescheduling
The TGA’s Interim Decisions against Rescheduling
The TGA Announced its Interim Decisions against Rescheduling on 21 October 2022.
Our Media Release in response to the TGA Interim Decision:
Use of Psychedelic Therapies for Treatment Resistant Patients Again Rejected by Government, Despite Strong Supporting Safety and Efficacy Data and Australia’s Worsening Mental Health Crisis
Read our media release here - 28 October, 2022
Our Submission in Response to the TGA Interim Submission 2022:
Our Submission in Response to the TGA Interim Submission 2022
Submission Opposing the Interim Decisions not to amend the Poisons Standard in Relation to the Restricted Medical Use of MDMA and Psilocybine.
